Solid Tumors
 The self-protective features presented by solid tumors are more difficult to overcome than the roadblocks presented by blood tumors. It’s a major reason why adoptive cell therapy is further along for blood cancers than for many solid tumors.
The self-protective features presented by solid tumors are more difficult to overcome than the roadblocks presented by blood tumors. It’s a major reason why adoptive cell therapy is further along for blood cancers than for many solid tumors.
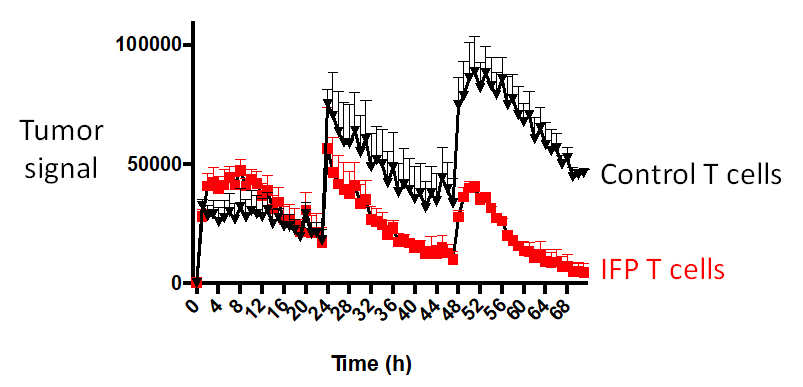
One of the barriers presented by solid tumors is T cell exhaustion. Normal T cells, as well as T cells that are modified with adoptive cell therapies such as CAR T, become exhausted before they are able to mount a sustained attack on tumor cells. Using IncuCyte technology, we are able to observe how the T cells modified with FPs behave over time in a setting that mirrors a human environment. This allows us to measure the durability of a T cell’s killing response to wave after wave of tumor cells. In mouse models of ovarian and pancreatic cancers, mice with our FP-enhanced cell therapy were able to destroy repeated additions of tumor cells, whereas control T cells became dysfunctional and ineffective.
 The “shut off” message that solid tumor cells send tells the T cells to self-destruct. The cancer cells even scatter the deadly messages at the edge of blood vessels, so some of the T cells die before they can reach the tumor. The FP we’ve crafted for our solid tumor studies rewires the T cells to interpret the negative message as one that stimulates their cancer-killing abilities.
The “shut off” message that solid tumor cells send tells the T cells to self-destruct. The cancer cells even scatter the deadly messages at the edge of blood vessels, so some of the T cells die before they can reach the tumor. The FP we’ve crafted for our solid tumor studies rewires the T cells to interpret the negative message as one that stimulates their cancer-killing abilities.
We have engineered a FP that combines a death receptor ectodomain, Fas, with the pro-survival costimulatory 4-1BB signaling domain, converting a death signal to an activation boost for T cells. We showed that the Fas-41BB FP supported improved survival in murine models of AML, ovarian cancer and pancreatic cancer. We are excited to determine the tumor microenvironment obstacles that FPs can overcome and extend these findings to create effective tumor immunotherapies.