Projects
Genetic Risk for Type 1 Diabetes
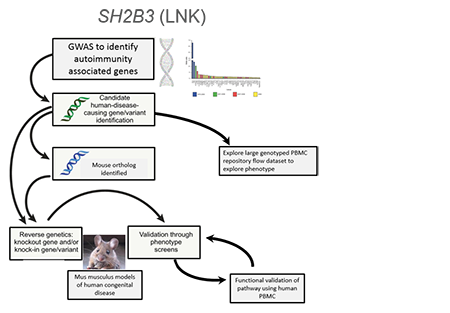 A common genetic variant (present in at least 5% of a population) can get enriched get enriched to help fight a specific microbe (infection) or adjust to a new change in the environment. This same variant may also have unintended side effects like autoimmune disease. We combined a genetic association study comparing subjects with Type 1 Diabetes and controls with similar genetic backgrounds and looked through all common variants across our population to find those enriched for Type 1 diabetes. We discovered a strongly associated variant (rs3184504) that located in the gene SH2B3, encoding the adaptor protein SH2B3 (also termed LNK). This adaptor dampens down cytokine signaling and highly expressed in blood cells. The genetic region was enriched (selected) in Northern European populations and nearly absent in African and Asian populations that all carry the “ancestral allele” or the allele not associated with diabetes, similar to non-human primates. Through studying primary blood samples from humans and developing a novel mouse model to study this risk during stress (infection) and in models of autoimmunity, the project goal is to better understand how this critical adaptor protein shapes the immune response in the presence of this precise genetic variant.
A common genetic variant (present in at least 5% of a population) can get enriched get enriched to help fight a specific microbe (infection) or adjust to a new change in the environment. This same variant may also have unintended side effects like autoimmune disease. We combined a genetic association study comparing subjects with Type 1 Diabetes and controls with similar genetic backgrounds and looked through all common variants across our population to find those enriched for Type 1 diabetes. We discovered a strongly associated variant (rs3184504) that located in the gene SH2B3, encoding the adaptor protein SH2B3 (also termed LNK). This adaptor dampens down cytokine signaling and highly expressed in blood cells. The genetic region was enriched (selected) in Northern European populations and nearly absent in African and Asian populations that all carry the “ancestral allele” or the allele not associated with diabetes, similar to non-human primates. Through studying primary blood samples from humans and developing a novel mouse model to study this risk during stress (infection) and in models of autoimmunity, the project goal is to better understand how this critical adaptor protein shapes the immune response in the presence of this precise genetic variant.
- Funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Understanding the Underlying Variants Leading to Autoimmunity or Predisposition to Infection
 Our patient-directed research aims to confirm and understand how particular genetic mutations can lead to disease. Rare genetic conditions are often characterized by early onset and severe symptoms, yet can be challenging to diagnose given limited knowledge or the uncharacterized nature of the disease. We apply next-generation sequencing paired with deep immunophenotyping approaches to aid in unsolved cases. By studying rare or early onset cases, we might accelerate diagnoses and/or acquire knowledge more broadly about the immune system.
Our patient-directed research aims to confirm and understand how particular genetic mutations can lead to disease. Rare genetic conditions are often characterized by early onset and severe symptoms, yet can be challenging to diagnose given limited knowledge or the uncharacterized nature of the disease. We apply next-generation sequencing paired with deep immunophenotyping approaches to aid in unsolved cases. By studying rare or early onset cases, we might accelerate diagnoses and/or acquire knowledge more broadly about the immune system.
Active projects:
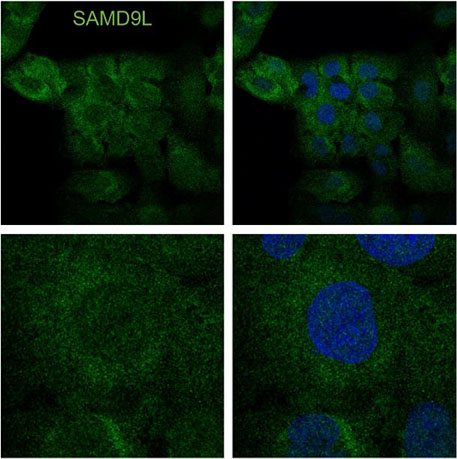
- Identify novel rare genetic variants that cause familial or individual immunodeficiency diseases such as SAMD9L-associated autoinflammatory disorder. SAMD9L is an interferon-induced tumor suppressor implicated in a spectrum of multi-system disorders including risk for myeloid malignancies and immune deficiency. This project has focused on understanding how a gain-of-function variant blocks mRNA translation and the role of SAMD9L as an antiviral protein.
- Funded by the Brotman Baty Institute.
- Funded by the Brotman Baty Institute.
- Ask new questions of known immune system disorders such as deeply studying a family with numerous siblings with RAG2-deficiency. RAG2 is an essential gene that when missing causes Severe Combined Immunodeficiency (SCID) or “boy-in-the-bubble” disease. However, less severe (hypomorphic) variants can lead to immune dysregulation and even autoimmune disease. In deeply studying blood samples from several affected siblings longitudinally over 30 years, we employed repertoire analysis of T cells and B cells to gain insight into the immune system with this perturbation.
- Read this article from the New York Times for more information about SCID.
- Read this article from the New York Times for more information about SCID.
- How do mosaic and somatic variants impact the immune system? Mosaic genetic changes are only carried in a portion of cells in the body occurring after the single cell (post-zygotic) stage. Somatic variants are thought to be acquired after birth. Having only a portion of your cells with the pathogenic variant can cause unique phenotypes. We are exploring the immune phenotype in patients with ALPS, or autoimmune lymphoproliferative syndrome (FAS gene), RALD, or Ras-associated lymphoproliferative disease (NRAS/KRAS genes) and reversion mutations in IL2RG gene. The detection of these changes is particularly challenging and can significantly alter the symptoms in each patient.