How to Decide to Join a Research Study
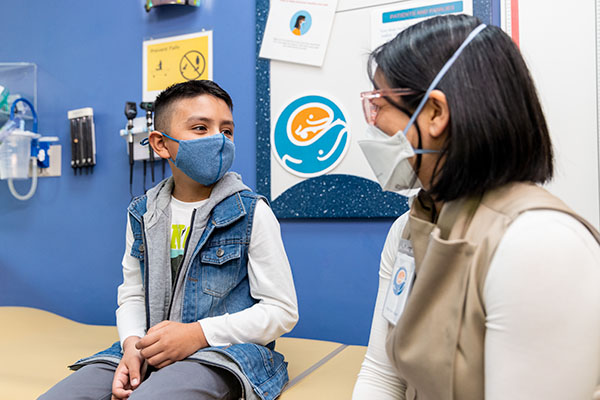 Choosing to join a research study is something you and your family can decide together. Before you make a decision, we will provide you with information about the study and answer any questions you might have.
Choosing to join a research study is something you and your family can decide together. Before you make a decision, we will provide you with information about the study and answer any questions you might have.Here are some tools to help you make the decision to participate in a study at Seattle Children’s.
Download our resource guides:
- Participate in Research – English (PDF)
- Participate in Research (Participar en la investigación) – Spanish (PDF)
- What Is Informed Consent? (PDF)
- Privacy in Clinical Research (PDF)
- What Is Clinical Research? (PDF)
Talking With Your Research Team
- Bring someone with you to the appointment for support and to help ask questions.
- Write down your questions before the visit and bring them with you. Don’t be afraid to ask the same question more than once until you feel your question has been answered.
- Take notes. This way you can review them whenever you want.
- Find out who to contact in case you have questions later.
- If your child seems to be having trouble understanding information about the study, ask about other ways we could present the information. We might be able to use videos or other visual aids.
- Ask for a language interpreter before or during your visit if you feel it would help you understand information better. A research team member can help arrange this.
Questions to Ask
Here are some questions you might consider asking before your child joins a research study. Most of these topics will be covered during your first research visit.
About the study
- Why are researchers doing this study? What do they hope to learn?
- Is there previous research on this medicine or treatment?
- Has the U.S. Food and Drug Administration (FDA) approved the medicine or treatment for use with people?
- Why do researchers think this might work?
- What are our responsibilities if my child takes part?
- Could being in this study affect my child’s daily life or that of my family?
- How long will the study last?
- Who will be responsible for my child’s medical care during the study?
- What kinds of procedures or tests will my child have during the study?
- Who do I contact if I have questions before, during and after the study?
- What happens if we want to stop being in the study?
- Can my child participate in more than one study at a time?
Benefits and risks
- What are the possible benefits of being in this research study?
- What are possible side effects or risks?
- What if my child’s health gets worse during the study?
Privacy and rights
- How will my child’s health information be kept private?
- Who will have access to my child’s information?
- Can I tell people about the study?
- Will the study results be given to us? If so, how and when?
Costs
- Do we get paid for participating?
- Are any personal costs such as meals or transportation paid for?
- What will we have to pay for?
- Who pays the medical bills if my child is injured by the medicine or treatment that is being studied?
Helpful Terms to Know
Assent
Children give assent when they agree to take part in research.
Children who are old enough to express that they want to be in a study usually sign an assent form or give verbal assent. Most children age 7 or older can understand basic information about research studies.
Banking or storing samples
Sometimes information or samples from study participants are stored for researchers to use in future studies. Researchers sometimes share information or samples with other study teams.
Consent (or parental permission)
Parents/guardians give permission when they agree their child can take part in research.
Parents/guardians sign a consent form to confirm that they understand the study and give permission for their child to participate.
Clinical trials
Research studies that explore if a medication, treatment or medical device is safe and effective for people.
Double-blinded studies
See below under Single-blinded and double-blinded studies.
Epidemiology
A branch of research that looks at patterns of illness and disease in groups of people. It tries to identify the causes of disease.
Inclusion/exclusion criteria
Each study has a list of requirements of who can and cannot participate. The items that allow someone to participate in a study are called inclusion criteria. The factors that do not allow someone to participate are called exclusion criteria.
For example, a study may need participants ages 3 to 7 who have had chickenpox. This would be the inclusion criteria for the study. The exclusion criteria would be:
- Children younger than 3
- Children older than 7
- Children who have not had chickenpox
People who match the exclusion criteria are not considered for participation in the study.
Informed consent
A process you and your child go through before joining a research study. This is to make sure you both understand the study’s purpose, benefits, risks and other options.
Institutional review board (IRB)
A group of people that might include doctors, researchers, patient support groups, pharmacists and other experts who review and approve research studies before they begin.
Placebo
A pill or liquid that looks like the medicine used in the study but does not treat anything.
Placebos are used during studies to help researchers understand the effect a medicine or treatment might have. Researchers compare the effects of the medicine and the placebo in the study. That way, they can determine the effectiveness of the new medicine and check for side effects.
Protocol
A carefully designed plan to keep study participants safe and answer specific research questions.
Principal investigator (PI)
The person in charge of the study. A PI is often a medical doctor.
Randomization
The process by which study participants are assigned to different study groups by random chance (similar to flipping a coin or pulling a name out of a hat).
Sometimes, the participant and research team do not know what study group the participant is assigned to, but they are able find out what group was assigned if they ever need to know for safety reasons.
Research coordinator
A person who works with the principal investigator and helps run the study. At Seattle Children’s, this person is called a clinical research associate (CRA).
Single-blinded and double-blinded studies
In single-blinded studies, participants do not know if they are getting the study medicine or treatment or if they are getting placebo, but the research team does.
In double-blinded studies, participants and the research team do not know if participants are getting the study medicine or treatment or if they are getting placebo.